Meet Sirona Dx at the AACR Annual Meeting in Orlando April 15-19, 2023
Join us at the AACR 2023 Annual Meeting at booth #3442 to learn about our unique spatial-omics service capabilities that span the latest technology platforms. Discuss the best single cell multi-omics approaches for deep immunophenotyping and characterization of the tumor microenvironment to drive biomarker discovery and uncover new opportunities for therapeutic intervention. We look forward to updating you […]
Lunaphore and CRO Sirona Dx collaborate to expand COMET offerings to US-based biotech and biopharma customers
LAUSANNE, Switzerland and PORTLAND, OR, USA– February 23, 2023 – 5:00 pm (CET) | 8 am (PST) – Lunaphore, a Swiss life sciences company developing technology to enable spatial biology in every laboratory, and Sirona Dx, a leader in multi-omics, single-cell analytical services, today announced a strategic collaboration in which Sirona Dx will become the first US-based Contract Research […]
Sirona Dx Announces Partnership with Scailyte for AI driven end-point specific single cell analysis
Scailyte, a single-cell biomarker discovery company and Sirona Dx, a leader in multiomic single-cell analytical services, announce a strategic partnership to accelerate the development of Advanced Therapies. The companies will create a powerful biomarker discovery engine, leveraging Sirona Dx technical and laboratory workflow solutions in combination with Scailyte’s AI-driven data analytics platform ScaiVision™ to serve […]
Ultivue Announces Co-Marketing Agreement with Sirona Dx for Multiplexed Tissue Biomarker Solutions
Ultivue Announces Co-Marketing Agreement with Sirona DX for Multiplexed Tissue Biomarker Solutions for Precision Medicine CAMBRIDGE, Mass. & PORTLAND, Ore.–(BUSINESS WIRE)–Ultivue, Inc. an industry leader in multiplexing tools and novel image analysis solutions for tissue biomarker studies, and Sirona Dx, a technical contract research organization (CRO), providing advanced, single cell proteomics and genomics services to […]
Sirona Dx recognized as a Top 10 CRO of 2021 by Medhealth Outlook
The development of innovative immunotherapy approaches for cancers and autoimmune disorders among others, remains a top priority for Biopharma however numerous challenges create hurdles to progress. To begin with, there is a requirement for multi-faceted, high-parameter, single cell readouts from limited sample inputs to identify informative biomarker signatures. Furthermore, the complex nature of the underlying […]
About Sirona Dx
Sirona Dx is a CLIA accredited technical CRO, founded to accelerate the pace of immunotherapy and targeted therapy development. Bridging silos between life science tools developers and translational clinical research, our laboratory offers specialized high complexity single cell proteomics and genomics services to support drug discovery and development programs.


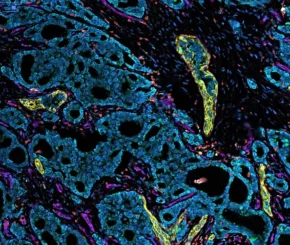




The powerful CosMx platform is now integrated at Sirona D
Pioneers of technology agnostic, spatial biology services since 2018. Our experienced team is here to help. Contact us for a free consultation to figure out the best approach for your spatial biology study. NanoString is honored to partner with Macrogen Inc., Propath UK, and Sirona Dx to accelerate access to spatial biology capabilities for biopharma companies around […]