
Sirona Dx is a technical CRO and leading provider of single cell multi-omics and spatial biology services. We support the design, optimization and analysis of complex, multiplexed assays within our GCLP and CLIA accredited laboratory. Our scientific and regulatory teams work closely to advance biomarker discovery and accelerate the development of next generation therapies for our clients worldwide.
In partnership with innovative life science platform developers, Sirona Dx executes a bold mission of enabling biopharma with leading-edge service capabilities. We embrace breakthrough technologies at an earlier stage and develop the deep expertise to harness them effectively for our clients.
Our specialized service capabilities for single cell and spatial biology are complemented by advanced approaches for bulk cell analysis.
Single Cell Multi-Omics and Spatial Biology Services
Technology Agnostic Spatial Biology Services
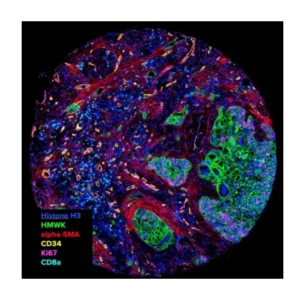
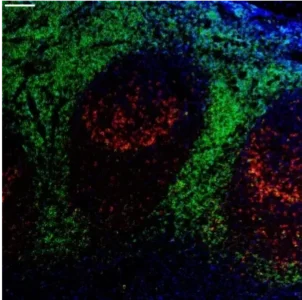
Sirona Dx launched the first spatial biology CRO service in 2018 and are recognized as pioneers in this rapidly evolving field. Today our integrated suite of spatial-omics technologies includes Imaging Mass Cytometry (Standard BioTools), PhenoImager (Akoya), COMET (Lunaphore) and CosMx (Nanostring). Sirona Dx remains technology agnostic and client specific study requirements will always guide platform selection in this rapidly evolving field.
Reveal the spatial dynamics and interplay of cell populations within the tumor microenvironment, which has been shown to be increasing important for therapeutic development.
Ultra-Deep Immunophenotyping with CyTOF / Mass Cytometry and CYTEK Full Spectrum Flow
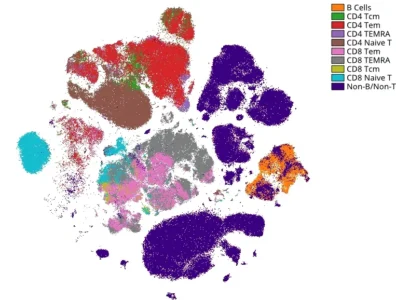
We harness CyTOF and CYTEK platforms to take immunophenotyping to the next level with panels as large as 45 markers. Core panels include Standard BioTools, Maxpar® Direct™ Immune Profiling Assay™ and our in-house designed and validated 31 marker Immune-Phenotyping panel. Assays can be customized to meet your study requirements and robustly validated to support clinical studies at our GCLP laboratory. Identify numerous cell subsets and characterize intracellular targets simultaneously within a single sample. Identify cell populations predicting therapeutic response and/or pharmacodynamic changes.
